Abstract
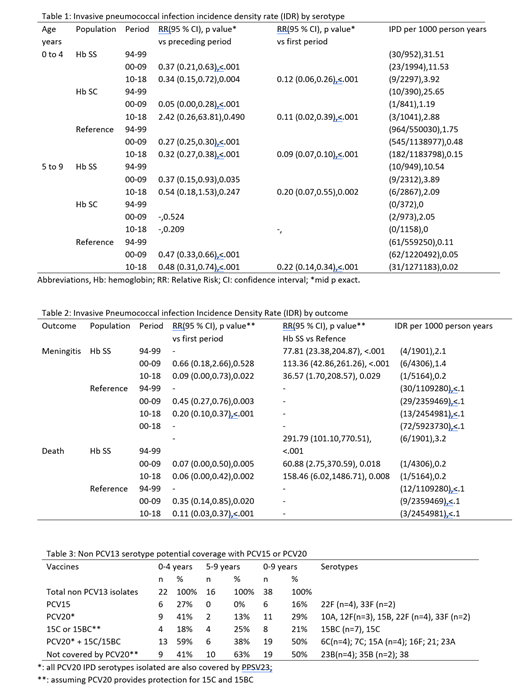
Before prophylactic antibiotic use, approximately 1/10 children <5 years old with sickle cell disease (SCD) developed invasive pneumococcal disease (IPD) with a high risk of meningitis and death. Although the emergence of penicillin-resistance in IPD threatened benefits of penicillin prophylaxis, after licensure of the 7-valent pneumococcal polysaccharide vaccine (PCV7) in 2000, IPD rates in children with SCD decreased by over 2/3. In 2010, PCV13 (serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 19A, 19F, 18C, 23F) was licensed in children, and in 2021, two additional conjugated vaccines were approved for use in adults: PCV15 (VAXNEUVANCE®, PCV13+: 22F, 33F) and PCV20 (PREVNAR20®, PCV13+: 8, 10A, 11A, 12F, 15B, 22F, 33F). IPD rates in children with SCD over 24 years in Atlanta were evaluated to describe trends in age of infection, frequency of antibiotic resistance, non-vaccine IPD serotype distribution after vaccine licensures, and estimates for serotype coverage by newer vaccines. IPD among children with SCD, ages 0 to 4 years and 5 to 9 years, residing in the Metropolitan area of Atlanta, Georgia, USA, from 1/1/1994 through 7/31/2018, were compared to the general population. The Centers for Disease Control and Prevention (CDC)-funded Georgia Emerging Infections Program (GA EIP) Active Bacterial Core Surveillance network included initially 8 counties in 1994 and from 1997 onward, 20 counties (total populations of approximately 3.8 and 5.1 million, respectively). Two registries of patients with SCD seen at least once at one of 3 pediatric hospitals serving the region were merged and matched with GA EIP data. The serotyping and antibiotic susceptibility testing were conducted at the CDC. The study was approved by the Emory University Institutional Review Board. Data from 3 periods: pre PCV period (94-99), PCV7 period (00-09) and PCV13 period (10-18) were analyzed. Compared to the pre PCV period, overall, IPD rates decreased in children with SCD vs 91% in the RP for 0-4 years; and 80% in children with SCD vs 78% in the RP for 5-9 years (table1). The difference in IPD rates between patients with SCD and the general population increased over time: pre PCV period, relative risk (RR)=24.22 (95 % Confidence Interval [CI] 17.43,32.88), P<.001; PCV 7 period, RR=32.17 (95 %CI 22.17,45.37), P<.001; PCV 13 period, RR=39.18 (95 %CI 22.35,64.69) P<.001. Meningitis and deaths from IPD decreased significantly in all populations examined but remained significantly higher in patients with SCD compared to RP (table 2). The mean age at IPD diagnosis for the 3 periods examined increased both in SCD and in the RP. For those with SCD (n=50), pre-PCV7 period mean age was :2.7 standard deviation (+/-) 2.3 years; for the PCV 13 period: n=19; 3.7 years +/-2.2 years p= 0.0877; for the RP: Pre-PCV period: n=1025; 1.3 +/-1.6 years; PCV 13 period: n=213; 2.2 +/-2.3 years. Overall absolute IPD rates declined for all age groups examined (table 1). Prior to PCV7 licensure, IPD in patients with SCD were significantly less likely to be penicillin susceptible (MIC <0.06 µg/mL) compared to the RP: 41.9% (18/43) vs 60.0% (476/793) RR=0.70 (95% CI 0.49,1.00), p=0.025. This difference was no longer present after PCV licensure (PCV 13 period , SCD 52.9% [9/17], vs RP 48.6% [70/144]). Non PCV serotypes IPD rates increased after PCV7 licensure but remained stable after PCV13 licensure; 16% of non PCV13 serotypes during all time periods are included in PCV15, whereas PCV20 may cover between 29% and 50% depending on related serotypes cross protection (table3). Although significantly less frequent compared with pre-PCV-era, IPD can be severe in patients with SCD. Increase in IPD age was seen in both SCD and RP. Lower rates of penicillin non-susceptibility may may reflect lower exposure to penicillin prophylaxis. Newer vaccines may offer expanded coverage for children with SCD. Ongoing surveillance will help determine their effect. Vaccines that cover all serotypes are needed.
No relevant conflicts of interest to declare.